WISDOM TOOTH SURGERY - SERVICE EVALUATION DATA ENTRY
Dentists and surgeons who treat third molars may participate in this service evaluation. All data will be submitted directly via the RedCap platform. Users can register to access the RedCap platform by clicking on the registration link. (Registration can take up to 3 working days.)
Prior to recruiting patients, please contact your clinical effectiveness team to determine whether approval for this project is required. Users can access the RedCap database to enter data here: https://trials.nforc.co.uk/
Once you have completed a clinical form you can download the data to use as your operation notes.
Study status – The service evaluation aims to assess the current practice in relation to lower wisdom teeth and data collected may be used to inform future ethically approved research.
The surgical treatment of the third molar (wisdom teeth) is one of the priority areas identified by The British Association of Oral and Maxillofacial Surgeons (BAOMS) for research. Wisdom teeth may erupt normally into correct dental alignment and function. Sometimes they develop in non-functional or minimally functional positions. Impaction occurs when there is prevention of complete eruption due to lack of space, obstruction or development in an abnormal position. This may result in a tooth erupting partially or not at all. Impaction may be associated with pathological changes including infection in the overlying tissues, an increased risk of caries and periodontal disease in adjacent teeth, and cyst formation.
The Wisdom Tooth Surgery Service Evaluation
- To explore the impact of wisdom tooth treatment on patients using the service.
- To look at clinical effectiveness, safety, patient experience and patient satisfaction.
- To measure the standard of care and performance of your particular clinic.
- To inform changes that could improve the delivery of the service.
- To help plan future services for people with wisdom tooth problems.
Data collection has been designed to be quick and efficient. Most of the data entry is a tick box exercise.
The project is collecting data on the elective and non–elective management of third molars. The clinical form should be completed immediately after treating the patient. You should have all the information available to complete entry for that episode.
Elective Management
Questions will be asked on:
- Location of procedure
- Reason for removal
- Relevant medical history
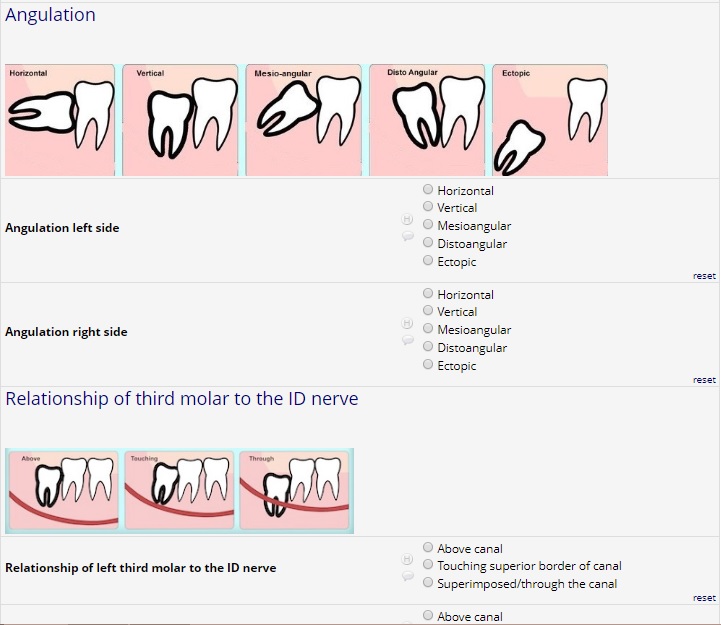
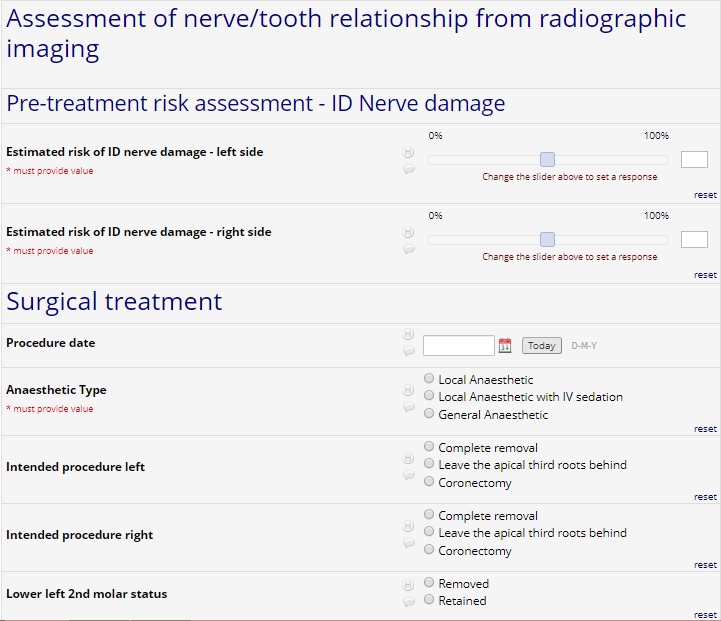
- Clinical/Radiograph position of third molar
- Planned procedure
- Untoward events
- Use of peri-operative adjuncts
Non–Elective Management
This section is to be completed if patients have presented with issues after having a third molar procedure or presented with an acute infection from a third molar that has not been treated before.
Follow up
At 3 weeks and 4 months, patients who had treatment for third molars will be contacted by email to complete a follow-up questionnaire. If the patient had a coronectomy and/or has long-term complications such as parenthesis and ongoing pain post-op they will be contacted at 12-months to complete another follow-up questionnaire. Your participation is not required at this stage, but please ensure their email address or telephone number is entered correctly.
Once you have completed a clinical form, all the data that you have entered will automatically self populate a comprehensive operation note that you can either cut and paste into your Electronic Patient record or place onto a word document to print out. The operation note will comprise of the indications for treatments, imaging, and treatments of lower third molars all from the data that you have selected. Although the primary objective is to study the third molar, other affected teeth that are treated at the same time could be documented too, such as the second molars. Please see the screen shot of the operation note below for an example.
To ensure we meet the strict information governance requirements we are using the Redcap database, which is run by The Barts and London along with Queen Mary University of London.
Registration is a three-step process
- Please register by filling out your details here. You will be sent a username and temporary password from a member of the NFORC team. This can take up to 1-2 working days.
- Go to the Barts & QMUL Helpdesk webpage and login with your details. Change your password to one more practical for you and enter your security questions.
- Go to RedCap https://trials.nforc.co.uk/ and log in with your username and new password. You’ll be sent a confirmatory email.
Prior to recruiting patients, please contact your clinical effectiveness team to determine whether approval for this project is required.
This service evaluation is funded by Saving Faces – The Facial Surgery Research Foundation and co-ordinated by the National Facial and Oral Research Centre (NFORC), a branch of Saving Faces.
Consultant in Oral and Maxillofacial Surgery & Sub Specialist Interest Group Lead for Dento-Alveolar & Oral Surgery – BAOMS
Mr Geoff Chiu
Professor of Oral and Maxillofacial Surgery & Honorary Consultant in Oral Surgery with Barts Health NHS Trust – BAOS
Prof Paul Coulthard
NFORC Director
Prof Iain Hutchison
Clinical Research Manager
Fran Ridout
We recommend that you print:
- a batch of Patient Information Sheets and Consent Forms in advance.
- the Information Sheet in a booklet format or 2 pages per sheet.
- the Consent Form double-sided.
The patient information sheet is for the patients to keep. Store signed consent forms in your patients’ notes.